Pioneering Intelligent Medicine with JKI
How cool would it be if your medication system could report your body's vital signs as well as your dosage history? Gary Palmer and his team at Proteus Biomedical are developing just such a system called Raisin™.
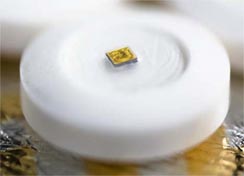
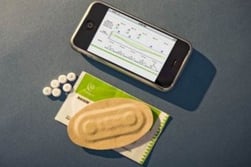
Raisin combines an intelligent pharmaceutical (a pill with a sensor) with a wearable monitor. Tiny, digestible sensors made from food ingredients and bonded to a patient's pills, then activated by stomach fluids after the patient swallows the pill. The patient also wears a small bandage-style patch on his or her skin to detect the ultra-low-power, private, digital signal emitted by the sensor.
This system can provide extremely useful data for healthcare providers and patients:
- The Raisin patch personal monitor records information such as type of drug, dose, and place of manufacture; captures date and time; and also measures and reports physiologic parameters such as heart rate and activity.
- Healthcare professionals can also derive metrics like sleep patterns and respiration rate from the collected data.
- The patch communicates with the patient's phone using Bluetooth, and the phone makes the data available to patients via user-friendly customized displays on mobile phones, tablets and desktop computers. Patients can choose who else can see their data, including family members or doctors.
Read the case study to see how Proteus and JKI created a collection of LabVIEW-based component and system testing tools to manage system verification and production testing for the Proteus Intelligent Medicine system. The result? With this amazing system, patients can better understand and manage their health. And doctors can provide better care because they have better information.
Note: The Raisin™ system is an Investigational Device limited by Federal (or United States) law to investigational use. The Raisin™ system is not for sale and is available for use only under Investigational Review Board (IRB) approved clinical studies.


Enjoyed the article? Leave us a comment